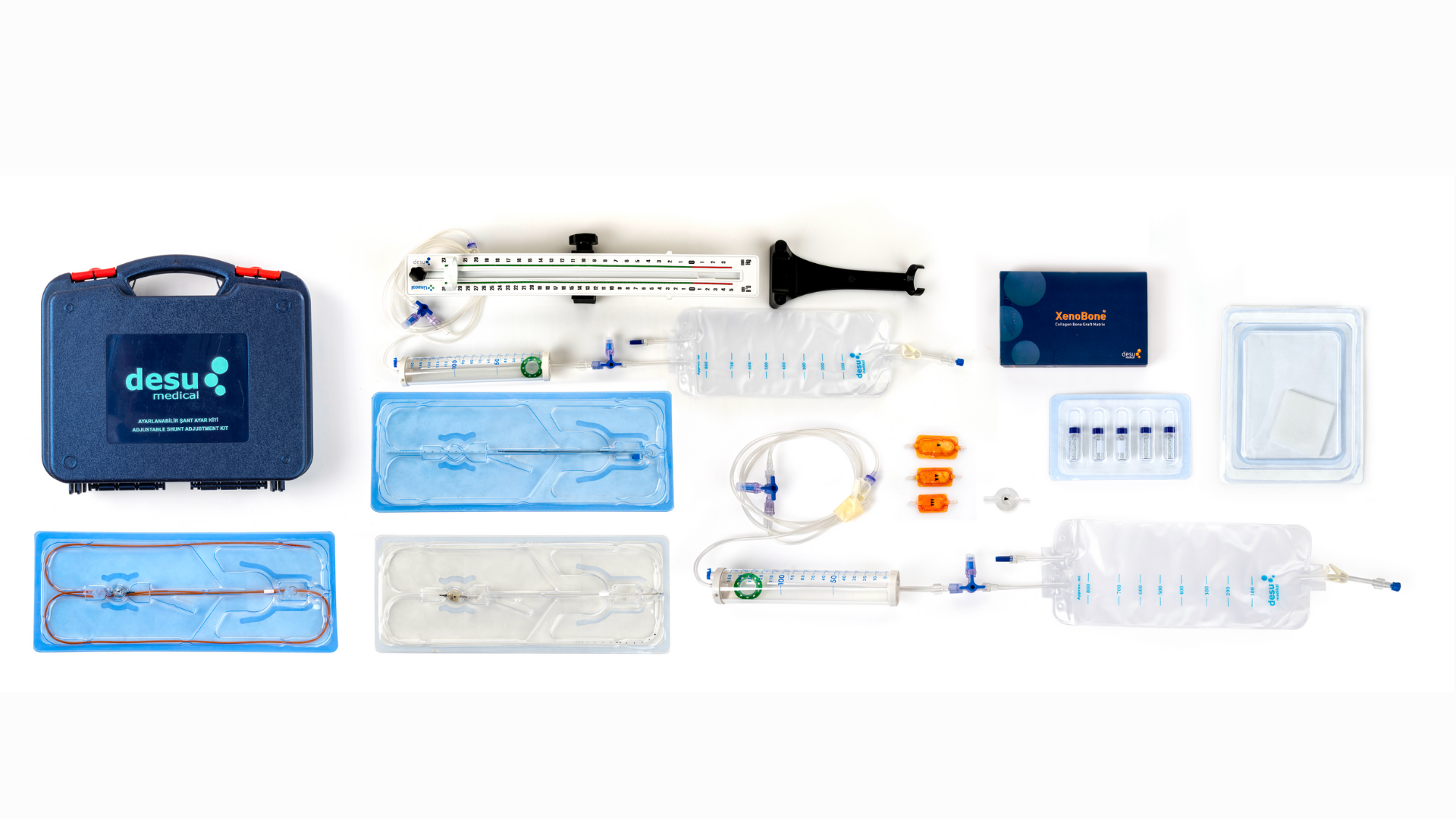
Silicone Shunt Kits
Hydrocephalus is a complex neurological condition characterized by an abnormal accumulation of cerebrospinal fluid (CSF) within the ventricles of the brain, often leading to increased intracranial pressure and potentially severe neurological impairment if left untreated. DESU Medical’s Silicone Hydrocephalus Shunt Kits are meticulously designed to offer a reliable and effective solution for managing this condition. These advanced systems integrate cutting-edge materials and innovative engineering to optimize performance, patient safety, and long-term outcomes.
Material Science and Composition
The components of DESU Medical’s shunt kits are constructed using polypropylene and silicone elastomer, two materials chosen for their superior properties:
- Biocompatibility: Both polypropylene and silicone elastomers are well-known for their excellent compatibility with human tissue, minimizing the risk of adverse immune responses and inflammation.
- Durability and Flexibility: Silicone elastomer offers the perfect balance of strength and flexibility, allowing the shunt system to adapt to the dynamic conditions of the human body without compromising structural integrity.
- Low Adhesion Properties: The surface characteristics of polypropylene and silicone elastomer significantly reduce the risk of adhesion or sticking, which can lead to valve deformation or malfunction over time.
The unique combination of these materials ensures that the shunt systems remain functional and reliable over extended periods, even in challenging physiological environments.
Advanced Valve Mechanism: Membrane-Based Design
At the heart of DESU Medical’s shunt kits is a membrane valve mechanism, a critical component that ensures precise regulation of CSF flow. This mechanism offers:
- Accurate Flow Control: The membrane responds to pressure fluctuations, allowing CSF to be diverted only when intracranial pressure exceeds a predetermined threshold. This prevents complications such as overdrainage, which can lead to subdural hematomas, or underdrainage, which may exacerbate hydrocephalus.
- Anti-Obstruction Properties: The design minimizes the risk of blockages, a common issue in traditional shunt systems, ensuring uninterrupted functionality.
- Consistency and Longevity: The membrane mechanism is engineered for long-term use, providing reliable performance over the lifespan of the device.
DESU® Antibiotic Shunt Kits – Enhanced Infection Control
Shunt infections are a significant concern in hydrocephalus management, often leading to severe complications, including meningitis and shunt failure. To address this, DESU offers DESU® Antibiotic Shunt Kits, which incorporate advanced antimicrobial features:
- Antibiotic-Impregnated Coating: The shunt components are coated with a broad-spectrum antibiotic that prevents the colonization of bacteria and biofilm formation, two primary causes of shunt-related infections.
- Sustained Release: The antibiotic is released gradually over time, providing long-lasting protection during the critical post-operative period.
- Reduced Antibiotic Resistance: By targeting the surgical site directly, these shunt kits minimize the need for systemic antibiotics, thereby reducing the risk of antibiotic resistance.
Clinical studies have demonstrated that the use of antibiotic-impregnated shunts significantly reduces infection rates, improving both short-term and long-term patient outcomes.
Applications and Versatility
DESU’s hydrocephalus shunt kits are suitable for a broad range of clinical scenarios:
- Congenital Hydrocephalus: Providing life-saving treatment for infants and children born with hydrocephalus, ensuring proper brain development and preventing neurological deficits.
- Acquired Hydrocephalus: Effectively managing conditions resulting from trauma, infection, tumors, or hemorrhage that lead to CSF buildup.
- Normal Pressure Hydrocephalus (NPH): Ideal for elderly patients experiencing NPH, a condition associated with gait disturbances, dementia, and urinary incontinence.
- Post-Surgical or Secondary Hydrocephalus: Addressing complications arising from brain surgeries or cranial pathologies that disrupt normal CSF dynamics.
Key Features and Benefits
- Material Excellence: Medical-grade silicone elastomer and polypropylene ensure biocompatibility, durability, and resistance to deformation. The use of low-adhesion materials minimizes the risk of sticking and valve malfunction.
- Membrane Valve Precision: Offers fine-tuned control of CSF flow, reducing the risk of overdrainage or underdrainage, and ensures consistent performance across a wide range of intracranial pressure scenarios.
- Reservoir for Monitoring: Facilitates non-invasive monitoring of shunt functionality and patient condition, allowing for straightforward sampling and pressure assessment during follow-up care.
- Infection Prevention with Antibiotic Kits: The DESU® Antibiotic Shunt Kits significantly lower infection rates, providing enhanced safety and long-term reliability.
- Customizability and Ease of Use: Available in various configurations to meet the specific needs of patients and surgeons. Easy to handle, implant, and adjust, reducing surgical complexity and operative time.
Clinical and Economic Benefits
The advanced features of DESU Medical’s shunt kits translate into both clinical and economic advantages:
- Improved Patient Outcomes: Reduced complications, faster recovery times, and enhanced quality of life for patients.
- Cost Savings: By minimizing infections, blockages, and the need for revision surgeries, these kits reduce overall healthcare costs.
- Surgeon Confidence: Reliable performance and ease of use allow surgeons to focus on delivering the best possible care.
Commitment to Quality and Innovation
DESU Medical is dedicated to advancing the field of neurosurgery through innovation and precision engineering. Our hydrocephalus shunt kits are manufactured in ISO-certified facilities under stringent quality control protocols, ensuring compliance with international medical device standards, including ISO 13485 and CE certification.
Conclusion
DESU Medical’s silicone-based hydrocephalus shunt kits, including the advanced DESU® Antibiotic Shunt Kits, are designed to meet the highest standards of safety, performance, and innovation. With their unique combination of biocompatible materials, precision-engineered valve mechanisms, and infection prevention technologies, these systems offer a comprehensive solution for the management of hydrocephalus.
Whether addressing congenital, acquired, or secondary hydrocephalus, DESU Medical shunt kits provide surgeons with the tools they need to achieve superior outcomes, while giving patients and their families renewed hope for a healthier future.
Choose DESU Medical for cutting-edge solutions that redefine the standard of care in hydrocephalus management.
Reservoir Structure for Enhanced Monitoring
An essential feature of DESU Medical’s shunt kits is the inclusion of a reservoir structure, which plays a pivotal role in monitoring and maintaining the shunt system. Key benefits include:
- CSF Sampling Capability: The reservoir allows clinicians to obtain cerebrospinal fluid samples directly, facilitating routine monitoring of shunt performance and early detection of infections or other complications.
- Pressure Assessment: The reservoir can be used to measure intracranial pressure (ICP), enabling healthcare providers to adjust the shunt system as needed for optimal patient outcomes.
- Ease of Access: The reservoir is strategically designed to be easily accessible during follow-up procedures, minimizing the need for invasive interventions.