Depus Shunts
Depus Shunts by Desu: Precision Solutions for Advanced Cerebrospinal Fluid Management
In the evolving landscape of neurosurgery, managing cerebrospinal fluid (CSF) conditions like hydrocephalus demands solutions that combine scientific rigor, safety, and long-term performance. The Depus Shunt system, developed by Desu, stands at the forefront of this field, offering unmatched versatility and reliability. Designed for both pediatric and adult patients, Depus Shunts deliver clinically proven outcomes in a wide range of CSF-related conditions. Whether it’s a simple fixed-pressure system or an advanced adjustable valve, Desu provides surgeons with the tools they need to succeed.
What is a Depus Shunt?
A Depus Shunt is a ventricular drainage system that redirects excess cerebrospinal fluid from the brain’s ventricles to other parts of the body, typically the peritoneal cavity. The system helps alleviate increased intracranial pressure caused by hydrocephalus, trauma, tumors, or idiopathic conditions. The core function of the shunt is to regulate fluid volume and pressure, preventing neurological damage and improving the patient’s quality of life. Desu’s Depus Shunt system is engineered for precision, featuring robust materials and intuitive designs that support consistent flow regulation over time.
Hydrodynamic Leverage Technology
What makes the Depus Shunt particularly unique is its internal hydrodynamic leverage mechanism, a passive yet responsive system that uses intracranial pressure itself to govern valve activity. At the heart of this mechanism is a finely balanced interaction between a titanium spring and a ruby ball. As CSF pressure rises, the spring compresses and the ball shifts, opening the valve. Once the pressure drops to normal levels, the valve closes, preventing further drainage. This ensures that the shunt only activates when needed, reducing risks such as overdrainage and ventricular collapse.
Superior Material: The Power of Polysulfone
Depus Shunts are constructed using medical-grade polysulfone, a thermoplastic polymer known for its biocompatibility, durability, and chemical resistance. Polysulfone’s transparency is also a significant advantage, as it enables visual inspection during surgery and follow-up. Unlike other materials that may degrade over time, polysulfone maintains its shape and performance inside the body for years. This translates to fewer revisions, greater reliability, and increased patient safety.
Multiple Valve Options for Clinical Flexibility
Desu offers a range of valve types under the Depus brand, making it easier for neurosurgeons to customize treatment:
Standard Valve: Designed for fixed-pressure regulation with consistent performance.
Adjustable Valve: Allows post-implantation pressure changes via a non-invasive magnetic tool.
Flat-Bottom Reservoir Valve: Engineered for smooth subcutaneous placement and anatomical fit.
Burr-Hole Reservoir Valve: Offers easy cranial integration during surgery.
Each type is available in pediatric and adult configurations, ensuring optimal compatibility with the patient’s anatomical and physiological requirements.
Pressure Configurations
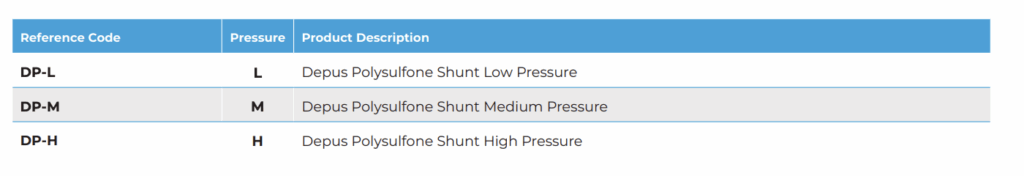
To further personalize treatment, Depus Shunts are manufactured in three pressure settings:
- Low Pressure (DP-L) – Suitable for infants and patients requiring minimal drainage.
- Medium Pressure (DP-M) – The standard choice for most adult and pediatric cases.
- High Pressure (DP-H) – Ideal for severe hydrocephalus or post-traumatic conditions.
These pressure levels ensure that drainage occurs at the appropriate rate, avoiding both under-treatment and over-correction.
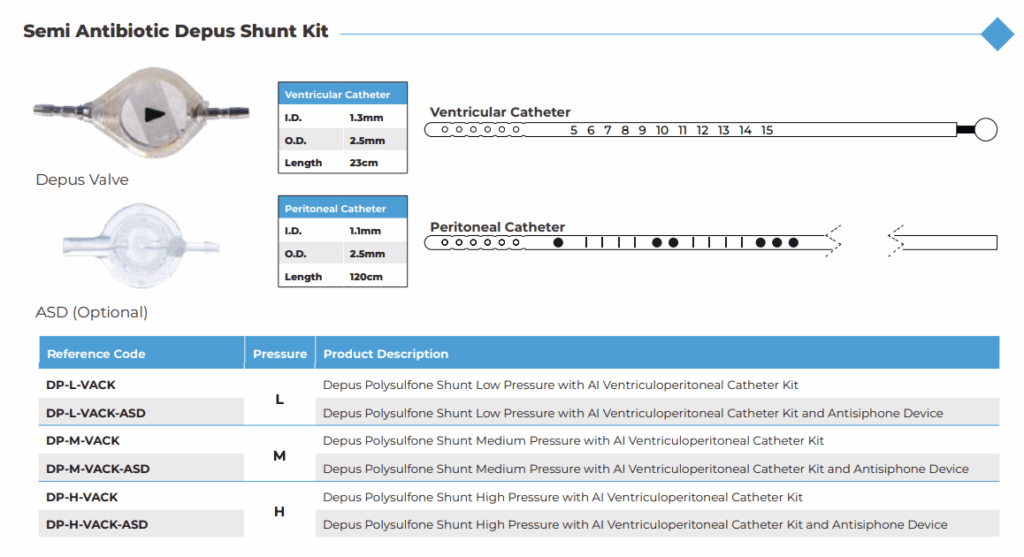
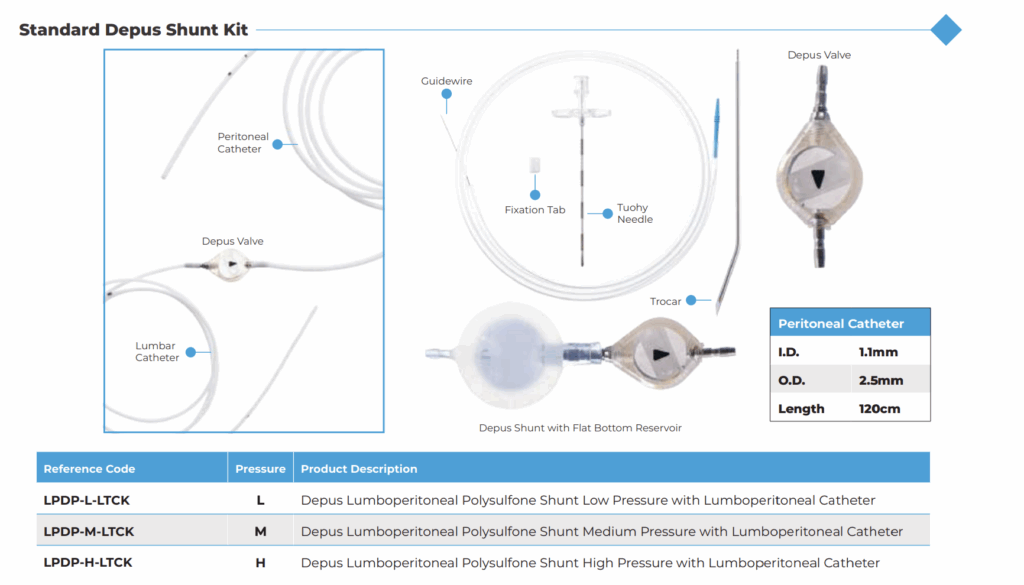
Complete Ventriculoperitoneal Shunt Kits
Desu also provides comprehensive ventriculoperitoneal shunt kits, streamlining surgical procedures by offering all necessary components in a sterile, ready-to-use format. Each kit includes:
- Depus Valve
- Ventricular Catheter (1.3 mm ID / 2.5 mm OD / 25 cm length)
- Peritoneal Catheter (1.1 mm ID / 2.5 mm OD / 120 cm length)
- Sağ Açılı Konnektör
- Stilet
- Optional Reservoir (Flat-Bottom or Burr-Hole)
- Optional Antisiphon Device (ASD)
These kits eliminate guesswork, reduce surgical preparation time, and ensure optimal component compatibility.
Semi Antibiotic Shunt Kit
Desu’s commitment to infection prevention is further reflected in its Semi Antibiotic Depus Shunt Kits. These kits include antimicrobial coatings on catheters to reduce bacterial colonization and minimize post-operative infection risks. This makes them particularly valuable in pediatric applications and for immunocompromised patients. Despite the added protection, performance remains on par with standard Depus kits, offering both safety and efficiency in one package.
Pediatric and Adult Applications
The Depus product line is designed with anatomical versatility in mind. Pediatric shunts have shorter, narrower components that accommodate smaller cranial volumes, while adult shunts are structured to handle greater fluid volumes and higher flow rates. Adjustable pressure options are especially beneficial in children, whose CSF dynamics can evolve as they grow. This age-specific engineering allows Depus Shunts to serve across a lifetime of care.
Antisiphon Device (ASD) and Reservoir Options
For patients at higher risk of postural overdrainage, Desu offers an Antisiphon Device (ASD). This device stabilizes the CSF flow when patients move from lying down to standing, which helps prevent complications such as subdural hematomas. Reservoir options—either flat-bottom or burr-hole—make post-operative monitoring and CSF access easier, enhancing both safety and follow-up care.
Clinical Indications
Depus Shunts are used in a wide range of clinical scenarios, including:
- Congenital hydrocephalus
- Acquired hydrocephalus following brain trauma or surgery
- Normal Pressure Hydrocephalus (NPH) in elderly patients
- Tumor-induced CSF blockage
- Idiopathic Intracranial Hypertension (IIH)
Their modular design and adaptability allow for tailored interventions across all age groups and medical contexts.
Trusted Performance Backed by Desu
Desu has built a reputation on scientific precision, manufacturing excellence, and real-world clinical success. All Depus products are manufactured under ISO and CE certifications, ensuring strict compliance with global safety and quality standards. Desu’s collaboration with leading neurosurgeons informs each product iteration, combining field expertise with biomedical innovation. The result is a shunt system that doctors trust and patients depend on.
The Depus Shunt System is more than just a medical device—it’s a complete solution for managing cerebrospinal fluid with intelligence, safety, and adaptability. Whether treating a newborn with congenital hydrocephalus or an adult with post-traumatic pressure buildup, Depus offers the right combination of engineering and clinical insight. Backed by Desu’s commitment to innovation, quality, and support, the Depus Shunt is the preferred choice for modern neurosurgeons worldwide.

